Process Analytical Technology in Biopharmaceutical.
I choose to learn from the best. When it comes to learning how to write better, UWriteMyEssay.net Dissertation On Process Analytical Technology is that company. The writers there are skillful, humble, passionate, teaching and tutoring from personal experience, and exited to show you the way.
Process Analytical Technology in Biopharmaceutical Manufacturing by Samuel T. Cosby B.S. Chemical Engineering Brigham Young University, 2007 Submitted to the Department of Chemical Engineering and the MIT Sloan School of Management in partial ful llment of the requirements for the degrees of Master of Science in Chemical Engineering and.

We present a methodology for evaluating the kinetic parameters of biomass transformations employing process analytical technology (PAT). The salient feature of this strategy involves the determination of reaction rates and activation energies for biomass conversions under process conditions without influence from possible side reactions or fouling of optical probes.

ABSTRACT OF DISSERTATION SIMULATIONS-GUIDED DESIGN OF PROCESS ANALYTICAL SENSOR USING MOLECULAR FACTOR COMPUTING Many areas of science now generate huge volumes of data that present visualization.

Process Analytical Technology Approach on Fluid Bed Granulation and Drying Identifying Critical Relationships and Constructing the Design Space Tanja Lipsanen ACADEMIC DISSERTATION To be presented, with the permission of the Faculty of Pharmacy of the University of Helsinki, for public examination in Auditorium 2, at Viikki Infocenter, on November 8th 2008, at 12 noon. Helsinki 2008.

Currently I am working for Vertex Pharmaceutical as a Manager, Technical Operations playing a key role in Process Analytical Technology team supporting method life cycle management and novel.

Process analytical technology (PAT) is a technique for the analysis and control of chemical processes to assure the production of acceptable quality of the end-product. In this thesis, the application of mid-IR (1900 - 650 cm -I) spectroscopy as a real-time monitoring technique for reaction and purification is illustrated. A technique using in.

Search this site: Humanities. Architecture and Environmental Design; Art History.

In this chapter, the main processing steps and manufacturing aspects of solid dosage forms are described and the relevant literature is reviewed. Starting with powder feeding, powder blending.
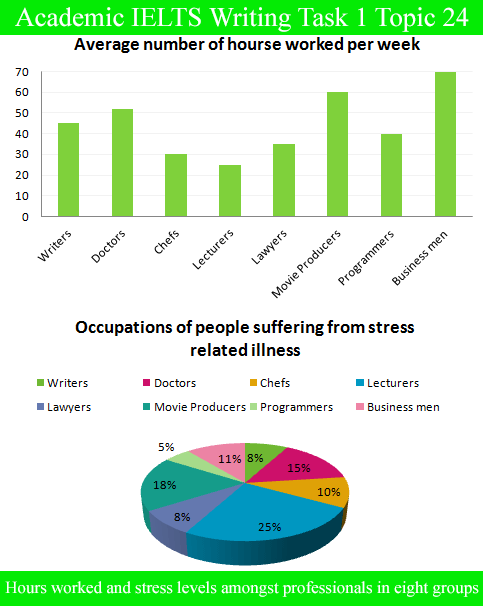
PAT Implementation in Pharmaceutical Manufacturing and its Economical Impact. The overall aim of this thesis was to evaluate the feasibility of implementing Process analytical Technology (PAT) into pharmaceutical manufacturing. First of all the implementation of PAT was looked at from a bird perspective, taking all unit operations into account. This task was divided into two parts, the.

It is clear that real-time quality assessment and control is indispensable for continuous production by means of Process Analytical Technology (PAT) tools. In this dissertation, the strengths and weaknesses of several complementary PAT tools, implemented in a continuous wet granulation process, which is part of a fully continuous from powder-to.
Process Analytical Technology and Chemometrics (15 credits) - describes online process monitoring and the use of Process Analytical Technology (PAT) to advance pharmaceutical process identification, simulation and control. Experimental Design and Research Methods (15 credits) - focuses on research and experiment design methods applying QbD.

Focus on the evaluation of Process Analytical Technology (PAT) for Upstream and Downstream Processing in Biopharmaceutical Manufacturing. Aspects of research include.